Hyperbaric Oxygen Therapy (HBOT)
Harnessing the Power of Oxygen to Promote Cellular Health
Hyperbaric Oxygen Therapy (HBOT) is a non-invasive treatment that enhances the body’s natural healing processes by delivering concentrated oxygen in a pressurized environment. This therapy significantly increases the amount of oxygen in the blood, allowing it to be absorbed by damaged tissues at a much higher rate than under normal atmospheric conditions.
Scientific Foundation: Nobel Prize Research on Oxygen Sensing Mechanisms
The 2019 Nobel Prize in Physiology or Medicine was awarded to scientists William Kaelin, Sir Peter Ratcliffe, and Gregg Semenza for their groundbreaking discovery of how cells sense and adapt to oxygen availability. This research reveals the fundamental mechanisms by which cells regulate gene expression in response to oxygen levels—a process critical for cellular repair, survival, and overall health.
HBOT directly supports the body’s oxygen-sensing systems, helping restore normal cellular function in hypoxic (low oxygen) environments, which are often associated with chronic diseases, injury, and impaired healing.

The Science Behind
Hyperbaric Oxygen Therapy (HBOT)
Oxygen deficiency activates the HIF (Hypoxia-Inducible Factor) signaling pathway, triggering various changes:
At the cellular level: HIF alters the expression of genes related to metabolism, cell survival, and death. This shifts the cells’ survival mode, leading to insufficient energy (ATP) production and an increase in harmful reactive oxygen species (ROS).
At the systemic level: Prolonged hypoxia elevates chronic inflammation throughout the body, accelerating inflammatory aging and the progression of chronic diseases. Continuous inflammation overstimulates the immune system, leading to immune dysfunction. In cancer patients, hypoxic environments in tumors promote cancer cell survival, migration, and disease progression.
By periodically replenishing oxygen in tissues and organs, hyperbaric oxygen therapy inhibits the HIF pathway, improves cellular metabolism, and enhances overall health.

Oxygen Regulation
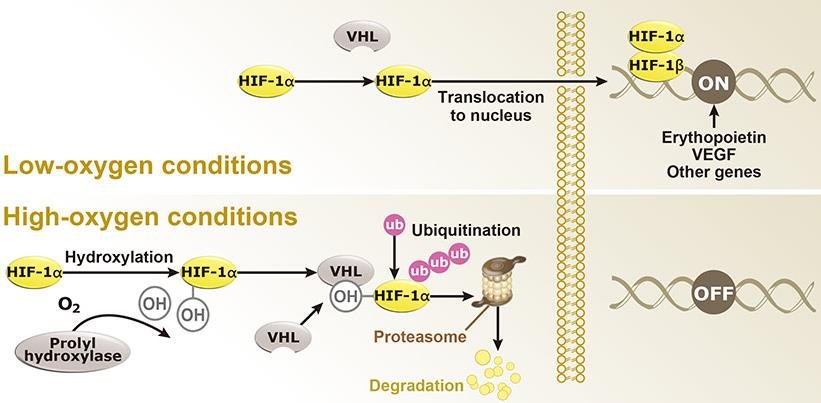
The core of the oxygen sensing and adaptation pathway is the HIF-1 protein (Hypoxia-Inducible Factor-1), which activates a range of hypoxia-responsive genes in animal cells. These include VEGF (Vascular Endothelial Growth Factor) and EPO (Erythropoietin). The proteins expressed by these genes stimulate red blood cell production and vascular growth, helping the body acquire more oxygen.
Another protein, VHL (Von Hippel-Lindau), plays a regulatory role by interacting with HIF-1α based on oxygen levels, modulating its quantity.
-
In low oxygen conditions: HIF-1α remains intact and enters the cell nucleus, where it combines with HIF-1β to initiate gene expression, promoting the hypoxia response.
-
In normal oxygen levels: Prolyl hydroxylase uses molecular oxygen (O₂) to add a hydroxyl group (OH) to HIF-1α. This allows VHL to bind to HIF-1α, tagging it with ubiquitins for proteasomal degradation. Without HIF-1α, hypoxia-inducible genes remain inactive.
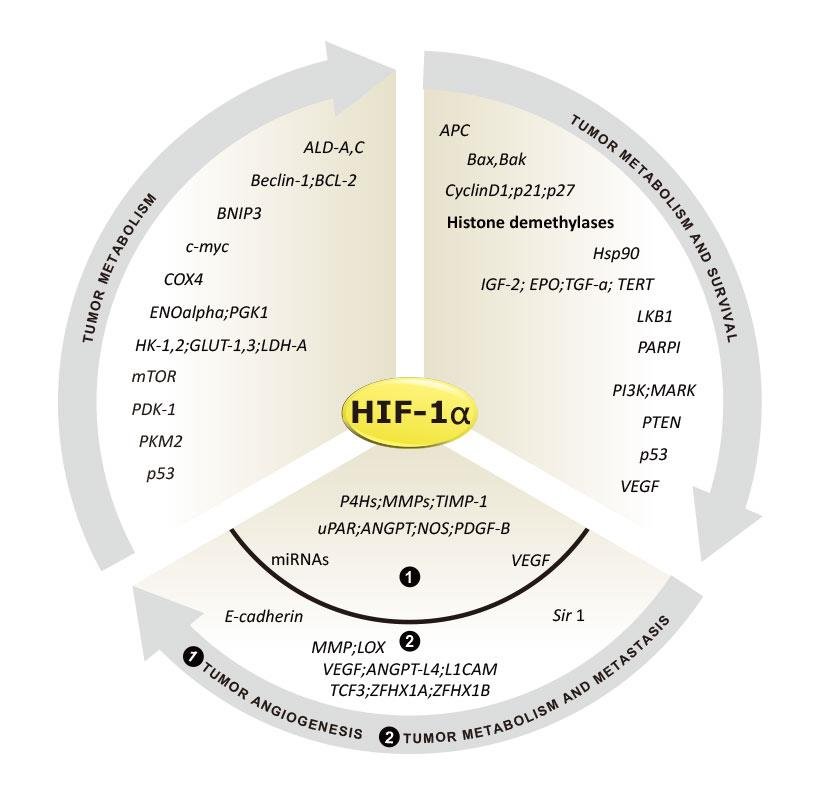
HIF-1 Regulation of Cancer-Related Genes
In many types of cancer, rapid proliferation of cancer cells often leads to localized oxygen deprivation around the tumor. This hypoxic environment limits the oxygen available to tissues or cells, prompting cancer cells to increase the expression of the HIF-1α protein. This elevation stimulates angiogenesis, the growth of new blood vessels, to supply the tumor with more oxygen and nutrients. This enables cancer cells to survive in low-oxygen environments, promoting their growth, invasion, and metastasis. It also triggers an inflammatory response in the body and makes cancer cells highly resistant to chemotherapy and radiotherapy.
Additionally, various gene mutations can further elevate HIF-1α expression, including gain-of-function mutations in oncogenes (such as ERBB2) and loss-of-function mutations in tumor suppressor genes (such as VHL and PTEN). Genes regulated by HIF-1 are closely tied to tumor metabolism, proliferation, survival, metastasis, and angiogenesis. As a result, inhibiting the function of HIF-1 and its related protein, HIF-2α, has become a significant focus in cancer drug development.